In this activity students will explore the nature of the ozone layer that protects the surface of the earth from ultraviolet radiation of the sun. They will find out how ozone is produced in the upper atmosphere, and what appears to be causing the deterioration of the earth's protective layer. Students will discuss the ways of curbing this trend, and in the extensions, explore a way to detect total column ozone levels.
spray can, set of Styrofoam spheres to make molecular models (or a variety of colors of clay), light meter, UV detector 1. Begin the lesson by showing a spray can product, and spraying a small amount into the air.Then say: "Some of the chemicals in spray cans can effect the ozone layer. How can this be? "Give students a few minutes in their groups to discuss what they know about this statement, and what might be creating the problem. 2. Explain to students that over millennia, that a layer of ozone (O3) has been built up by the interaction of photons of ultraviolet light and oxygen (O2) as shown in this reaction which you might refer to as the ozone formation cycle. Point out that the ozone in the stratosphere ozone absorbs ultraviolet radiation, and accomplishes two things: a. Shields the Earth from the dangerous ultraviolet photons, and b. Participates in the ozone formation cycle in which more ozone is produced even when an a ozone molecule is broken down when it absorbs photons.
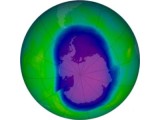
3. Explain to students that the concentration of ozone in the stratosphere is very small---if it could be compacted and brought to the surface of the earth, it would be a layer about 3 mm thick. Explain to students that chemicals produced by human activity has led to the endangerment of the ozone layer. Of greatest concern are molecules known as CFCs (chlorofluorocarbons). CFCs are chemicals that have been used in hair sprays, refrigerants, and cleaning fluids. Chemically they are very stable, and consequently, they do not have to be replaced (in refrigerators) very often. However, if they are released into the environment, they will remain there for a long time. CFCs that drift into the stratosphere react with ultraviolet photons releasing atoms that attack ozone. In the late 1970s, scientists reported this fact, and predicted a depletion of the amount of ozone if large amounts of CFCs drifted into the stratosphere. In the mid 1960s, using ground based and satellite measuring devices, scientists determined that the total column ozone over the Antarctic had decreased by 40% in a single year. This led to the concept of an "ozone hole," which has reappeared again since the mid 1960s. In the stratosphere, the reaction that concerns scientists is as shown in figure 6.16. A photon of ultraviolet light (UV) breaks the bond holding one of the chlorine atoms to the CFC. The chlorine is released and it reacts with ozone, destroying the ozone, and creating another molecule that can in turn react with oxygen to produce free chlorine which can attack another ozone molecule. Thus an individual CFC molecule can end up destroying many ozone molecules. 4. Use the Web to extend your understanding of ozone in the stratosphere. Here is an ozone sampler, a collection of sites that will help you understand ozone depletion, the ozone hole, what causes ozone depletion, and how it can abated. Identify a question that you want to answer, then select one of the sites below to find information to help you explain your question and answer to someone else. Ozone Sampler Ozone Chemistry 5. To understand ozone chemistry, obtain a number of polystyrene foam spheres (or clay) and make the following: Use the spheres to replicate the reactions that: b. Describe the way ozone is destroyed in the atmosphere CFCs that drift into the stratosphere react with ultraviolet photons releasing atoms that attack ozone. In the late 1970s, scientists reported this fact, and predicted a depletion of the amount of ozone if large amounts of CFCs drifted into the stratosphere. The chlorine is released and it reacts with ozone, destroying the ozone, and creating another molecule that can in turn react with oxygen to produce free chlorine which can attack another ozone molecule. Thus an individual CFC molecule can end up destroying many ozone molecules. Display your models, and prepare an explanation for other members of your class. 1. Take the The Ozone Hole Tour: Establised by the Centre for Atmospheric Science at Cambridge University, England, this site will help you learn about the ozone hole from a leading scientific establishment. 2. Some people do not agree with many scientific centers, governments and universities that ozone is being depleted in the stratosphere. Visit the Critiques of Ozone Depletion site which contains articles and press releases challenging the scientific models and preditions about ozone depletion. The site is managed by The Science & Environmental Policy Project, a non-profit organization located in Fairfax, Virginia. 2. Northern Hemisphere students might want to collaborate with Southern Hemisphere stduents, especially students living in Australia and New Zealand. The problem of ozone depletion and the ozone hole is a problem very real to the Southern Hemisphere. Students might share information with each other, and agree to collaborate in the sharing of total column ozone data. 3. You might find it interesting explore ways at the personal, local, regional and global levels for dealing with the problem of ozone depletion. What actions might and should be taken, and why would they be effective?


a. Describe the way ozone is produced in the atmosphere
Project Ozone
An Inquiry into air quality
Site Navigation[Skip]
Questions? Email